A more complete picture of you.

What BioCertica customers are saying:
"The results have become a key factor in developing a weight loss protocol as weight management is crucial in Olympic Weightlifting."
- Zach Snyman 🏋️♂️ South African weightlifting record holder
Health & Fitness features
Predispositions:
Know your predisposition for Type 2 diabetes, Joint Injury, Inflammation and Methylation status.
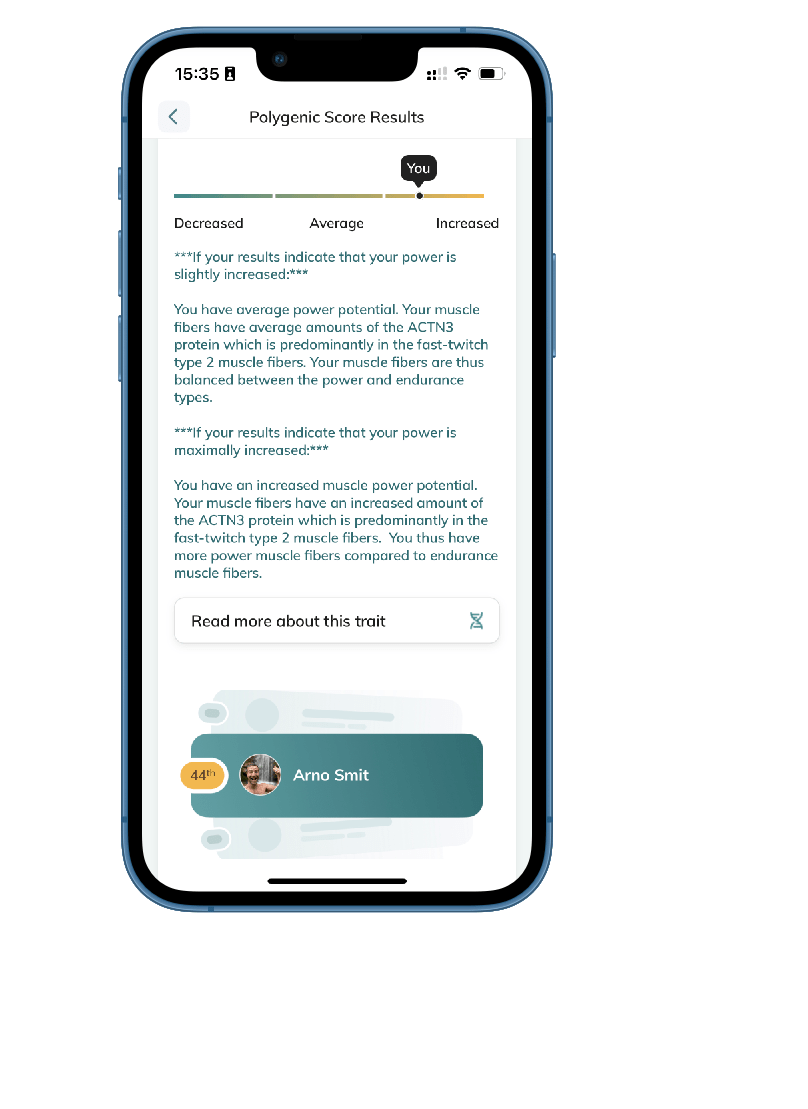
Fitness:
- Unleash Your Sprinter Gene Potential and Achieve Athletic Excellence with ACTN3 insights.
- Reach New Heights with VO2 Max and Growth Hormone Insights.
- Discover your Pain Sensitivity and Optimize Workouts.
- Unlock Your Genetic Potential for Lean Body Mass and Hand Grip Strength.
Ancestry features
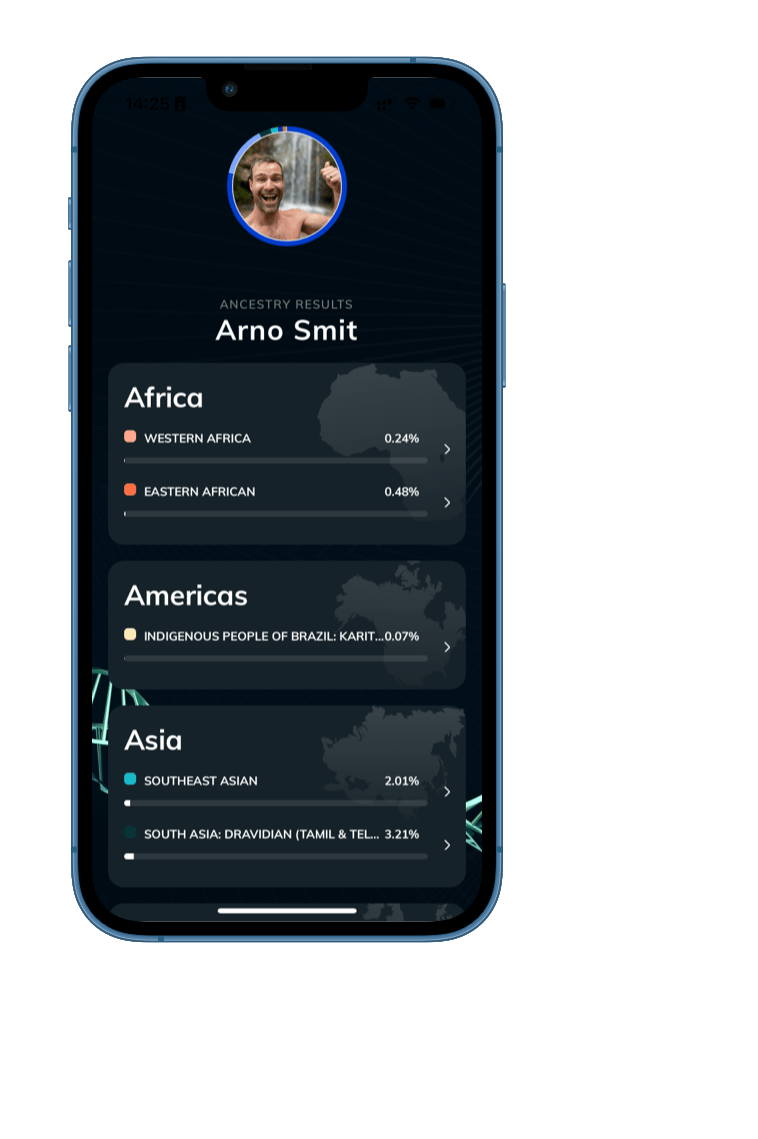
- Uncover Ancestral Insights - Gain unparalleled insights into your family's history and lineage.
- Celebrate Your Cultural Heritage - Gain a deeper connection to the traditions, customs, and vibrant history of your ancestors.
- Embrace Your Ancestry - Reveal the diverse ethnicities and connections that define your genetic heritage.
- Personalized Ancestry Reports - Receive comprehensive reports that beautifully capture the essence of your ancestral heritage.
Traits features
- Hair Loss Insights: Discover how your genes influence this trait and access valuable information to inform your hair care decisions.
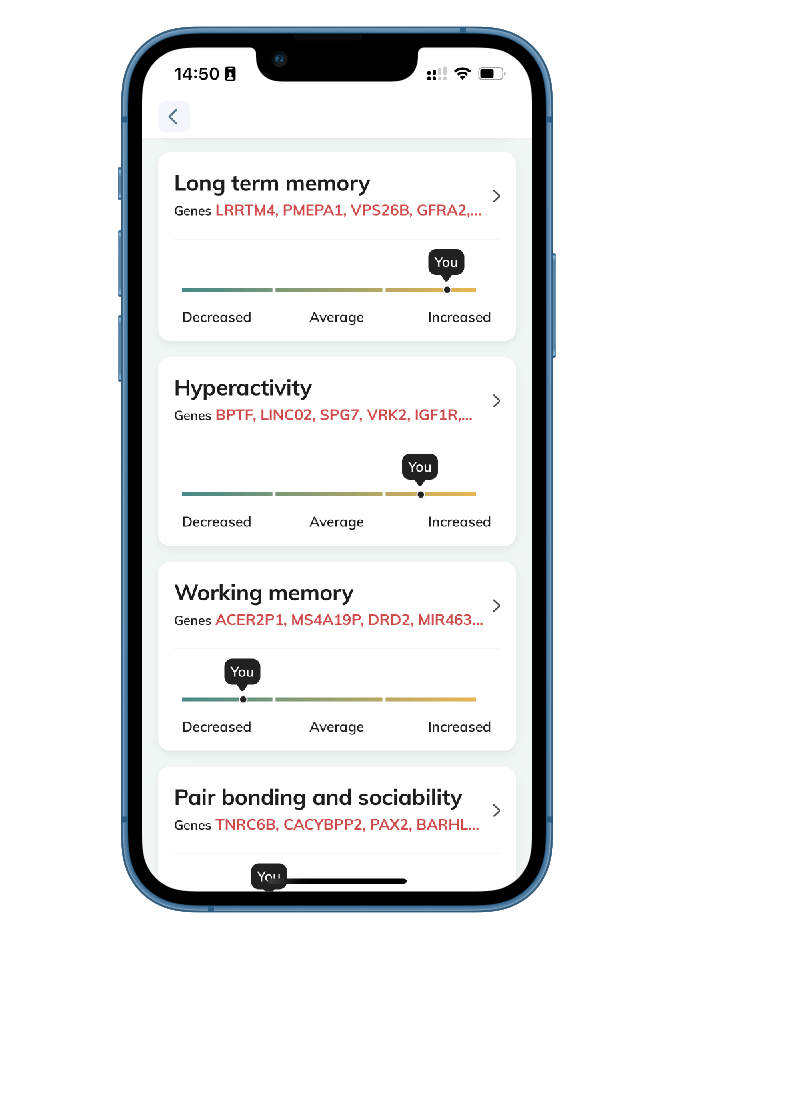
- Fascinating Behavioral Traits: Unveil insights into traits like body odor, memory, aggression, pair bonding, and sociability, and the fascinating interplay between your genes and your personality.
- Arthritis Risk Assessment: Learn about your genetic predisposition to arthritis and gain valuable knowledge to inform proactive measures for joint health.
- Personalized Reports: Receive comprehensive reports that interpret your genetic results and provide personalized insights based on your unique genetic makeup.
Frequently Asked Questions
How accurate are my test results?
Polygenic Risk Scores (PRS) offer a reliable estimation of an individual's likelihood to develop traits or conditions based on their genetic variants, derived from extensive genetic studies.
By aggregating the effects of numerous genetic markers linked to specific traits, PRS provides a comprehensive score. The accuracy of PRS predictions is influenced by the robustness and size of the genetic dataset, the strength of associations, and the complexity of the trait under consideration.
However, it's important to note that PRS doesn't encompass environmental factors or lifestyle choices, which are also contributors to disease risk. Nonetheless, PRS remains a valuable tool for offering insights into genetic predispositions.
What can I learn from getting my DNA tested?
Our approach, grounded in scientific research, involves analyzing 800,000 genetic markers. Through a process called polygenic risk scoring, we further enhance this data to encompass 2 million markers. This method draws upon extensive datasets to estimate the likelihood of various factors such as health conditions, cardiovascular challenges, fitness predispositions, and more.
The specific insights you gain are closely linked to the reports you choose to purchase. This comprehensive approach offers a deeper understanding of your genetic landscape and its potential implications.
How does the test work, and how long does it take?
1. Order Your Kit:
Begin by ordering your BioCertica DNA Kit online. Your kit will include a saliva collection tube and comprehensive instructions for easy and convenient use.
2. Collect Saliva at Home:
In the comfort of your own home, provide a saliva sample following the provided instructions. The collection process is simple and painless, ensuring your comfort every step of the way.
3. Send Your Sample:
Once you've collected your saliva sample, securely package it and send it back to us using the prepaid shipping label included in your kit. This step is designed to be hassle-free, ensuring your sample reaches our state-of-the-art laboratory intact.
4. Expert Processing:
Your saliva sample undergoes meticulous analysis at our cutting-edge laboratory. Our team of skilled geneticists and scientists decode the information contained within your DNA, unveiling the secrets of your ancestry and heritage.
5. Results at Your Fingertips:
Approximately 6-8 weeks after we receive your sample, your personalised results will be ready. Access them conveniently through our intuitive app. Delve into detailed reports that highlight your genetic background, including geographic origins and potential connections to diverse populations.
Embarking on this journey with BioCertica allows you to uncover the stories hidden within your genes, connecting you to the rich tapestry of humanity's history.
Start your adventure today and anticipate results that will reshape your understanding of your own origins. With BioCertica, the past and the present converge in a seamless, enlightening experience.
Note: The entire process, from ordering your kit to receiving your results, takes approximately 6-8 weeks, giving you ample time to eagerly anticipate the revelations that await you.
Who can test their DNA through BioCertica?
At BioCertica, we're dedicated to unlocking the mysteries hidden within your DNA to provide you with unparalleled insights into your origins, health, and well-being. Our DNA testing is tailored for individuals in South Africa, offering a unique opportunity to delve into your genetic heritage. Here's who can benefit the most from our cutting-edge DNA testing:
1. Those Curious About Their Roots:
If you've ever wondered about your ancestral origins, BioCertica DNA testing can reveal the geographic regions that contributed to your genetic makeup. Whether you're tracing your lineage or exploring the diverse cultures that have shaped you, our testing provides a window into your past.
2. Early Explorers for Enhanced Accuracy:
For the most accurate results, consider testing your DNA at an earlier age. The information encoded in your DNA becomes more refined over time, making earlier testing an ideal choice for those seeking a deeper understanding of their genetic background.
3. Enthusiasts of Holistic Well-being:
If you're passionate about optimising your nutrition, fitness, and overall health, our DNA testing offers comprehensive insights. Discover how your unique genetic makeup influences your body's response to different foods and exercises, helping you tailor your lifestyle choices for maximum well-being.
4. Individuals Managing Chronic Conditions:
Our pharmacogentics reports are a boon for those dealing with chronic health conditions. Understand how your genetics can impact your response to medications, enabling you and your healthcare provider to make more informed decisions about your treatment plan.
Empower yourself with knowledge that's not only informative but also transformative. Join the BioCertica community and embark on a journey of self-discovery that combines science, heritage, and well-being like never before.
Why should I choose BioCertica?
We pride ourselves in the science of genetic testing, the rich history and data it holds, and believe that understanding your DNA is a pivotal part of longevity.

